

Over 130 million
people are now eligible for protection1,3,4
Nearly
3 of 4 acute hepatitis B cases in 2022 occurred in people with no risk factors identified5
It may be difficult to predict who can contract hepatitis B6

All eligible adults aged 19‑59
SHOULD receive hepatitis B vaccination

Adults aged 60+ with risk factors
SHOULD receive hepatitis B vaccination

Adults aged 60+ without known risk factors
MAY receive hepatitis B vaccination
Liver cancer is the third-leading cause of cancer deaths in the world8
of liver cancer cases worldwide are due to hepatitis B9
On average in the US, there are 42,000 new liver cancer cases and 30,000 deaths each year; not all cases are hepatitis B–related.10‡
† For most people, HBV clears on its own. But for those who don’t clear the virus, it can progress to chronic hepatitis B and potentially life-threatening consequences such as liver cancer. From 2018–2022, there were an estimated 13,000–22,000 cases of acute hepatitis B annually in the US.6,11,12
‡ Estimates for 2024 per the Surveillance, Epidemiology, and End Results Program (SEER) data set.
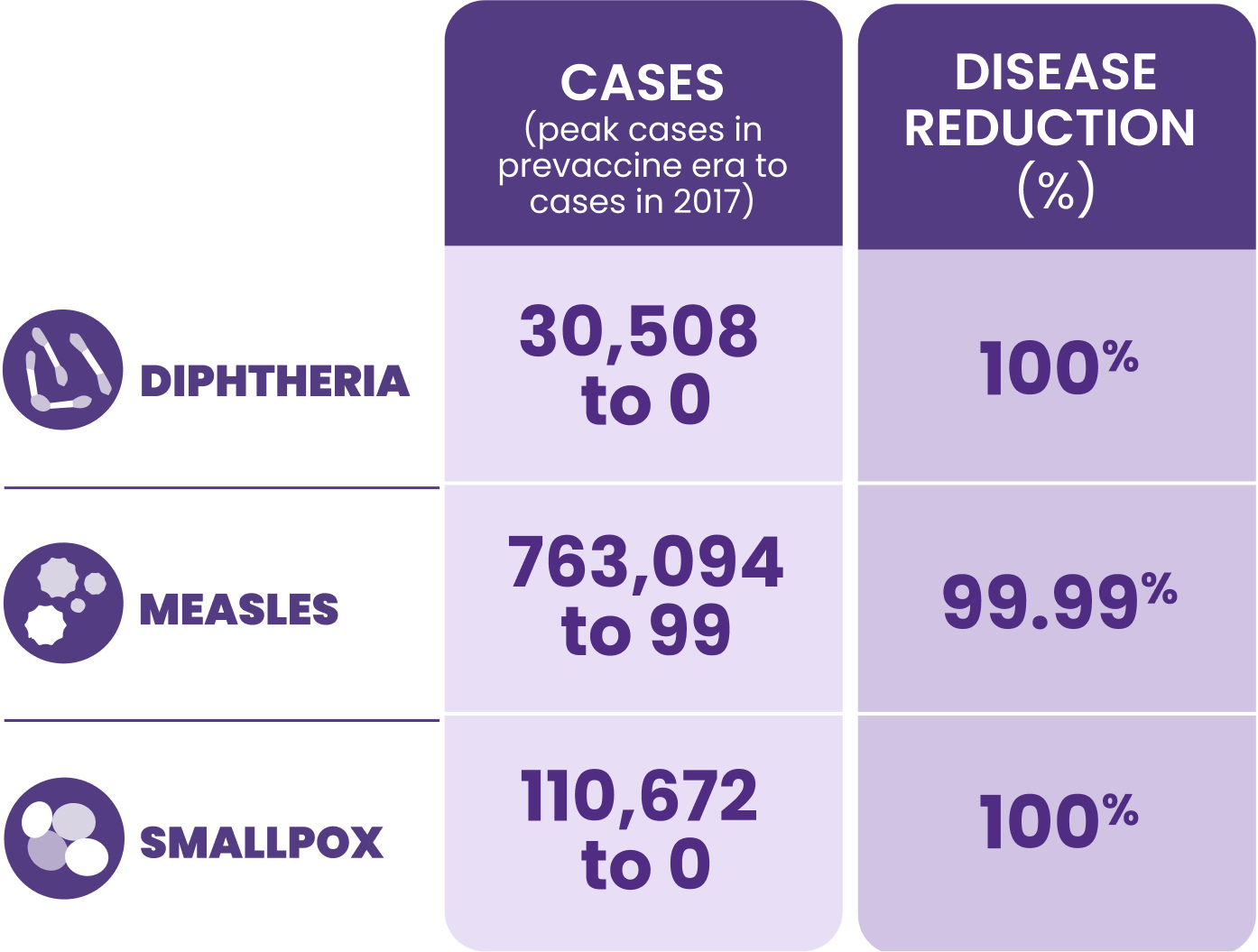
HEPLISAV‑B does not treat liver diseases such as cirrhosis or liver cancer.13
Although treatments are available, the best way to prevent it is through vaccination§
Together with the World Health Organization (WHO) and the US Department of Health and Human Services (HHS), we can help eliminate hepatitis B by 203014

WHO’s global hepatitis strategy, endorsed by all WHO member states, aims to reduce new hepatitis B infections by 90% and deaths by 65% by 2030.15

The Viral Hepatitis Elimination Plan provides a framework to control the viral hepatitis epidemic and eliminate viral hepatitis as a public health threat in the United States by 2030.14

||Born before 1991.

Help meet the WHO goal of elimination by 203015
*The ACIP recommends hepatitis B vaccination for all adults aged 19–59 and for adults aged ≥60 years with risk factors. Adults aged ≥60 years without known risk factors may receive hepatitis B vaccination. This recommendation does not apply to adults who have received a complete HBV vaccine series in their lifetime or have a history of HBV infection.1
§For most people, HBV clears on its own. But for those who don’t clear the virus, it can progress to chronic hepatitis B and potentially life-threatening consequences such as liver cancer. From 2018–2022, there were an estimated 13,000–22,000 cases of acute hepatitis B annually in the US.6,11,12
ACIP=Advisory Committee on Immunization Practices; CDC=Centers for Disease Control and Prevention; HHS=Department of Health and Human Services; WHO=World Health Organization
This site uses cookies to improve your experience. By continuing to use this website you are agreeing to our Cookie Policy.
INDICATION
HEPLISAV‑B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV‑B.
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to HEPLISAV‑B.
Hepatitis B has a long incubation period. HEPLISAV‑B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common patient-reported adverse reactions reported within 7 days of vaccination were injection site pain (23%‑39%), fatigue (11%‑17%), and headache (8%‑17%).
There are no adequate and well-controlled studies of HEPLISAV‑B in pregnant individuals. Available data, primarily in individuals who received one dose of HEPLISAV‑B in the 28 days prior to or during pregnancy, do not suggest an increased risk of major birth defects and miscarriage.
It is not known whether HEPLISAV‑B is excreted in human milk. Data are not available to assess the effects of HEPLISAV‑B on the breastfed infant or on milk production/excretion.
Vaccination with HEPLISAV‑B may not result in protection of all vaccine recipients.
ADDITIONAL IMPORTANT INFORMATION
HEPLISAV‑B does not treat liver diseases such as cirrhosis or liver cancer.13
Not all liver cancer is caused by the hepatitis B virus.18
Please see full Prescribing Information.
1. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483. doi:10.15585/mmwr.mm7113a1 2. Hall EW, Weng MK, Harris AM, et al. Assessing the cost-utility of universal hepatitis B vaccination among adults. J Infect Dis. 2022;226(6):1041-1051. doi:10.1093/infdis/jiac088 3. Data on file. Dynavax Technologies Corporation. Flow model for universal hepatitis B vaccination (version 4.5) assumptions. May 24, 2021. 4. Data on file. Dynavax Technologies Corporation. Hep B market map. Final readout. December 3, 2024. 5. Centers for Disease Control and Prevention. Availability of information on risk behaviors or exposures associated with reported cases of acute hepatitis B — United States, 2022. Updated October 21, 2024. Accessed January 24, 2025. https://www.cdc.gov/hepatitis-surveillance-2022/hepatitis-b/figure-2-7.html 6. Centers for Disease Control and Prevention. Clinical overview of hepatitis B. Updated February 9, 2024. Accessed January 28, 2025. https://www.cdc.gov/hepatitis-b/hcp/clinical-overview/ 7. Doshani M. Evidence to recommendations framework: should all HepB-unvaccinated adults receive hepatitis B vaccination? Presented to ACIP; September 29, 2021. 8. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598-1606. doi:10.1016/j.jhep.2022.08.021 9. Llovet JM, Ducreux M, Lencioni R, et al. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. 10. National Cancer Institute. Cancer stat facts: liver and intrahepatic bile duct cancer. Accessed August 14, 2024. https://seer.cancer.gov/statfacts/html/livibd.html 11. Centers for Disease Control and Prevention. Number of reported cases and estimated infections of acute hepatitis B — United States, 2015–2022. Updated October 21, 2024. Accessed January 23, 2025. https://www.cdc.gov/hepatitis-surveillance-2022/hepatitis-b/figure-2-1.html 12. Centers for Disease Control and Prevention. Hepatitis B surveillance 2022. Updated April 4, 2024. Accessed January 28, 2025. https://www.cdc.gov/hepatitis-surveillance-2022/hepatitis-b/index.html 13. HEPLISAV-B [package insert]. Emeryville, CA: Dynavax Technologies Corporation; 2024. 14. U.S. Department of Health and Human Services. Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021–2025). 2020. Washington, DC. 15. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021: Towards Ending Viral Hepatitis. June 2016. 16. Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11(1526):1-15. doi:10.3389/fmicb.2020.01526 17. He WQ, Guo GN, Li C. The impact of hepatitis B vaccination in the United States, 1999–2018. Hepatology. 2022;75(6):1566‐1578. doi:10.1002/hep.32265 18. National Cancer Institute. Liver cancer causes, risk factors, and prevention. Updated May 15, 2024. Accessed July 15, 2024. https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors