Series completion is critical to help ensure protective immunity2
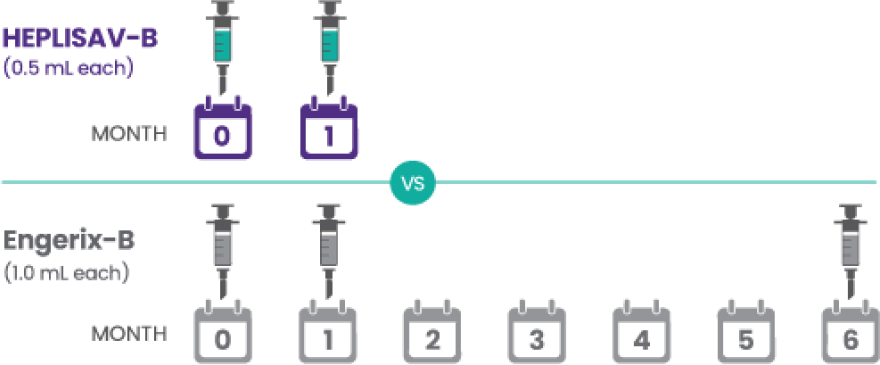
In a real-world study of >10k participants, a large healthcare system assessed whether recipients of a 2-dose hepatitis B vaccine are more likely to complete their series compared with recipients of a 3-dose vaccine and found that...

*This was a nested cohort study of 10,888 adult Kaiser Permanente Southern California (KPSC) members not receiving dialysis who received a first dose of the hepatitis B vaccine series in primary care settings. Patient data were captured from default electronic health record (EHR) order sets. Individuals were followed up through the EHRs for up to 1 year after the first dose to assess their receipt of subsequent doses of the HEPLISAV‑B or Engerix‑B vaccines.3
Applies to the percentage of individuals who completed the series within 1 year.3
Series completion within the recommended vaccine schedule plus 3 months (45% for HEPLISAV‑B and 26% for Engerix‑B) was the primary outcome and series completion within 1 year (61% for HEPLISAV‑B and 32% for Engerix‑B) after receipt of the first dose was the secondary outcome. Limitations: Results of this study may have limited generalizability in settings with greater proportions of underinsured or uninsured individuals or in the absence of electronic reminders for hepatitis B vaccination. Outcome misclassification could have occurred if hepatitis B vaccine doses were received outside of the KPSC system and not documented in the KPSC EHRs; this potential bias is likely minimal.3


fewer shots for your patients, staff, and practice3-5
(as compared to other hepatitis B vaccines)
ACIP-RECOMMENDED VACCINES ARE AVAILABLE WITH NO OUT-OF-POCKET COST TO THE PATIENT6†
Coverage and cost may vary and are subject to change without notice. Reimbursement decisions are made by individual insurance plans.
†The Advisory Committee on Immunization Practices recommends vaccines for CDC review and publication; all recommendations are eligible for first-dollar coverage.
This site uses cookies to improve your experience. By continuing to use this website you are agreeing to our Cookie Policy.
INDICATION
HEPLISAV‑B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV‑B.
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to HEPLISAV‑B.
Hepatitis B has a long incubation period. HEPLISAV‑B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common patient-reported adverse reactions reported within 7 days of vaccination were injection site pain (23%‑39%), fatigue (11%‑17%), and headache (8%‑17%).
There are no adequate and well-controlled studies of HEPLISAV‑B in pregnant individuals. Available data, primarily in individuals who received one dose of HEPLISAV‑B in the 28 days prior to or during pregnancy, do not suggest an increased risk of major birth defects and miscarriage.
It is not known whether HEPLISAV‑B is excreted in human milk. Data are not available to assess the effects of HEPLISAV‑B on the breastfed infant or on milk production/excretion.
Vaccination with HEPLISAV‑B may not result in protection of all vaccine recipients.
ADDITIONAL IMPORTANT INFORMATION
HEPLISAV‑B does not treat liver diseases such as cirrhosis or liver cancer.1
Not all liver cancer is caused by the hepatitis B virus.7
Please see full Prescribing Information.
1. HEPLISAV‑B [package insert]. Emeryville, CA: Dynavax Technologies Corporation; 2024. 2. Centers for Disease Control and Prevention. Hepatitis B basics. Updated January 12, 2024. Accessed July 12, 2024. https://www.cdc.gov/hepatitis-b/about/ 3. Bruxvoort K, Slezak J, Huang R, et al. Association of number of doses with hepatitis B vaccine series completion in US adults. JAMA Netw Open. 2020;3(11):e2027577. doi:10.1001/jamanetworkopen.2020.27577 4. Centers for Disease Control and Prevention. Recommended adult immunization schedule for ages 19 years or older. Updated November 21, 2024. Accessed January 23, 2025. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/adult/adult-combined-schedule.pdf 5. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(S2):S389-S397. doi:10.2105/AJPH.2008.151332 6. Hughes R IV, Maxim R, Fix A. Vague vaccine recommendations may be leading to lack of provider clarity, confusion over coverage. Health Affairs Blog. May 7, 2019. Accessed July 18, 2024. https://www.healthaffairs.org/do/10.1377/forefront.20190506.172246 7. National Cancer Institute. Liver cancer causes, risk factors, and prevention. Last updated May 15, 2024. Accessed July 15, 2024. https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors