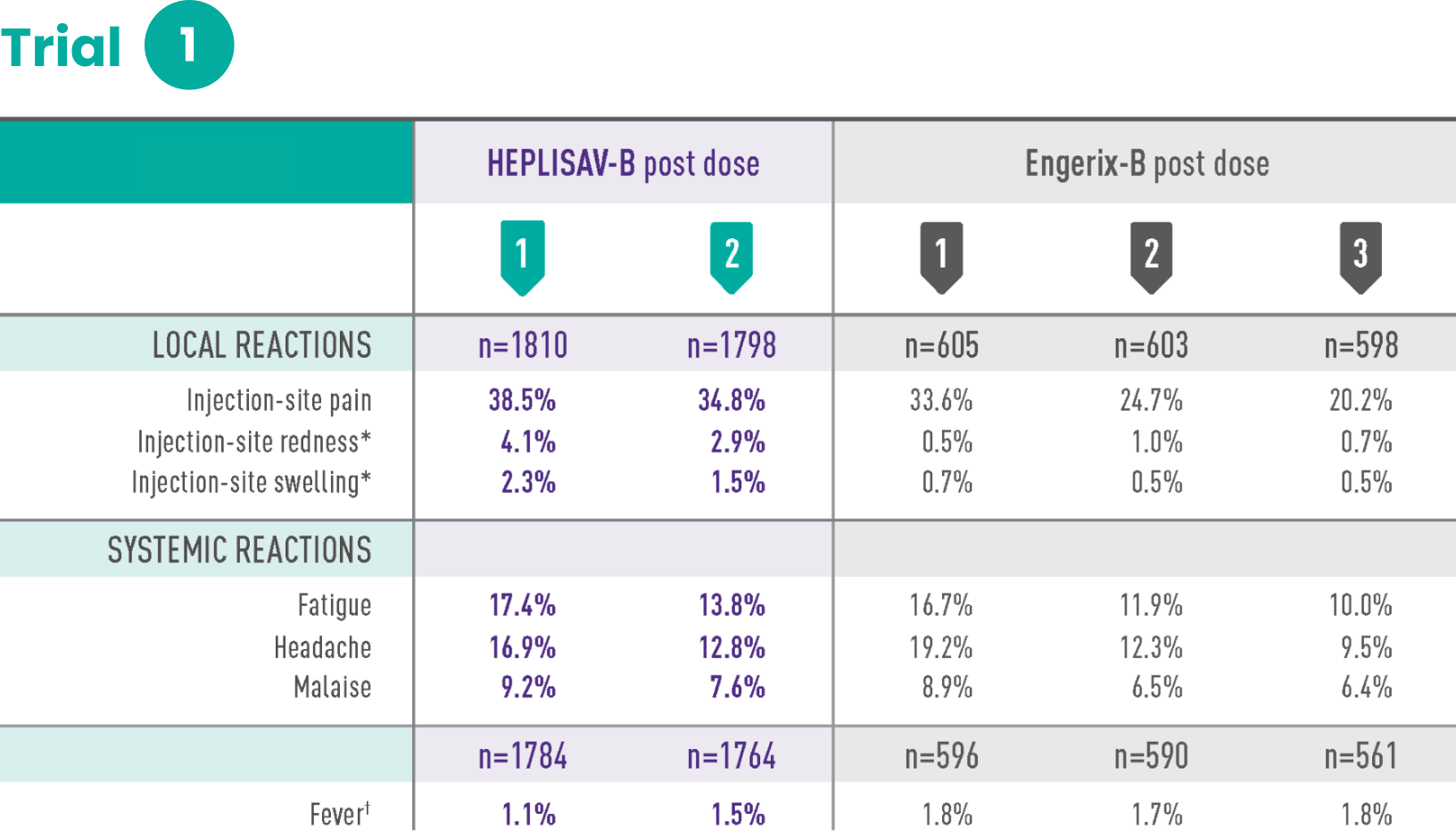
Study 1 was a randomized, observer-blind, active-controlled multicenter study in Canada and Germany in which 1810 subjects received at least 1 dose of HEPLISAV‑B and 605 subjects received at least 1 dose of Engerix‑B® [Hepatitis B Vaccine (Recombinant)]. Enrolled subjects had no history of hepatitis B vaccination or infection. HEPLISAV‑B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Engerix‑B was given at 0, 1, and 6 months. In the total study population, the mean age was 40 years; 46% of the subjects were men; 93% were white, 2% black, 3% Asian, and 3% Hispanic; 26% were obese, 10% had hypertension, 8% had dyslipidemia, and 2% had diabetes mellitus. These demographic and baseline characteristics were similar in both vaccine groups.
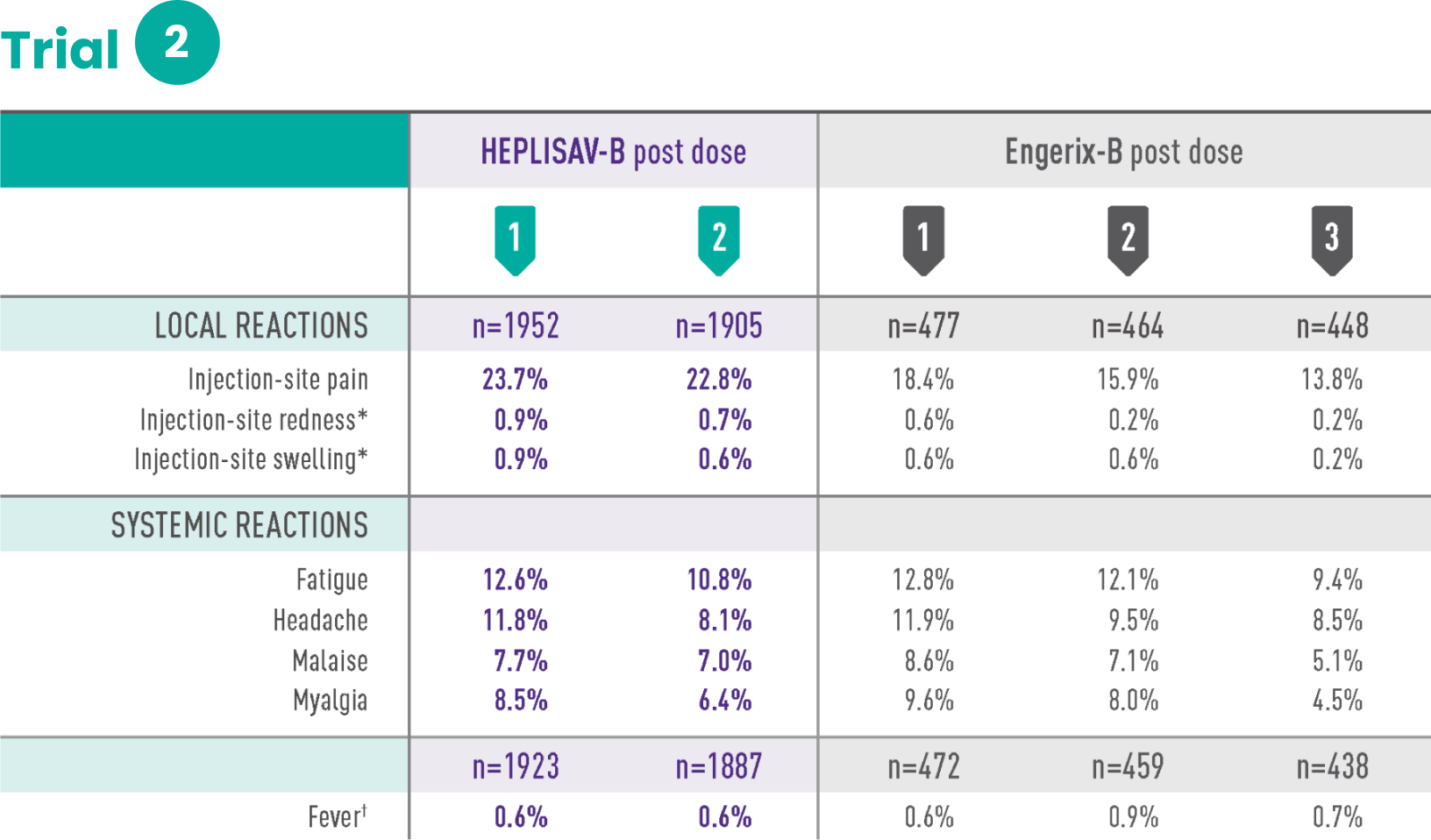
Study 2 was a randomized, observer-blind, active-controlled multicenter study in Canada and the United States in which 1968 subjects received at least 1 dose of HEPLISAV‑B and 481 subjects received at least 1 dose of Engerix‑B. HEPLISAV‑B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Enrolled subjects had no history of hepatitis B vaccination or infection. Engerix‑B was given at 0, 1, and 6 months. In the total population, the mean age was 54 years; 48% of subjects were men; 82% were white, 15% black, 1% Asian, and 6% Hispanic; 44% were obese, 30% had hypertension, 30% had dyslipidemia, and 8% had diabetes mellitus. These demographic and baseline characteristics were similar in both vaccine groups.
*Redness and swelling ≥2.5 cm.
†Oral temperature ≥100.4˚F (38.0˚C).
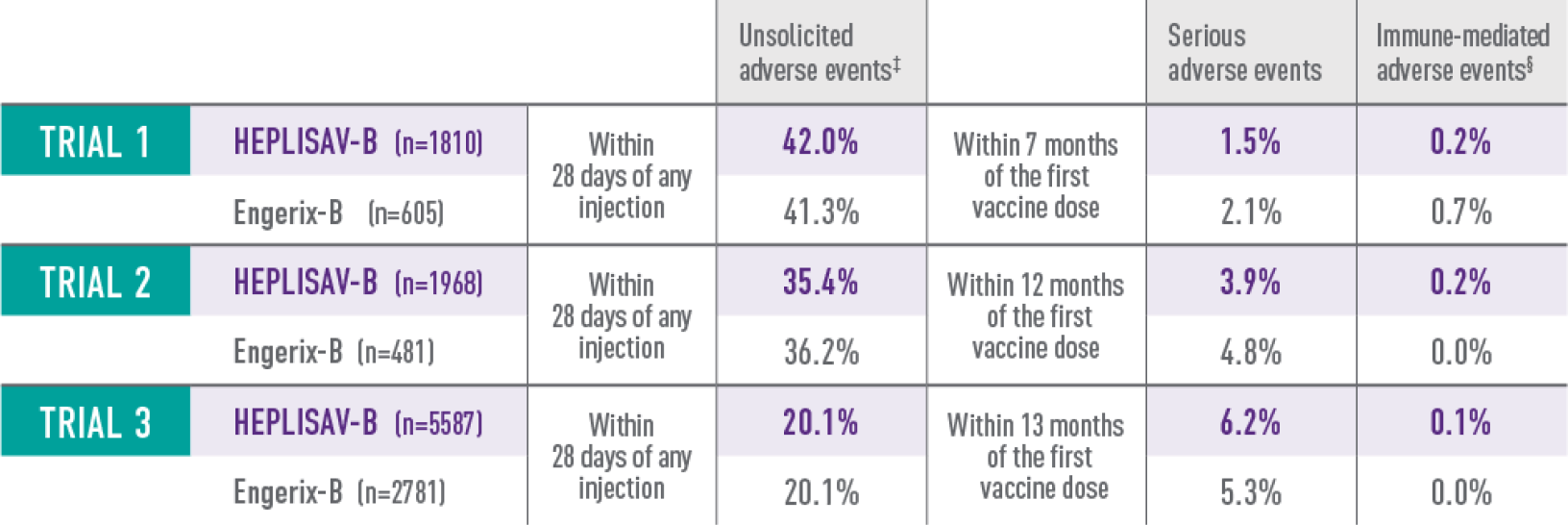
Study 3 was a randomized, observer-blind, active-controlled multicenter study in the United States in which 5587 subjects received at least 1 dose of HEPLISAV‑B and 2781 subjects received at least 1 dose of Engerix‑B. Enrolled subjects had no history of hepatitis B vaccination or infection. HEPLISAV‑B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Engerix‑B was given at 0, 1, and 6 months. In the total study population, the mean age was 50 years; 51% were men; 71% were white, 26% black, 1% Asian, and 9% Hispanic; 48% were obese, 36% had hypertension, 32% had dyslipidemia, and 14% had type 2 diabetes mellitus. These demographic and baseline characteristics were similar in both vaccine groups.
‡For trial 3, only unsolicited medically attended adverse events—those for which a subject sought medical care—were captured.
§For trials 2 and 3, new-onset autoimmune adverse events are listed.
This site uses cookies to improve your experience. By continuing to use this website you are agreeing to our Cookie Policy.
INDICATION
HEPLISAV‑B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV‑B.
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to HEPLISAV‑B.
Hepatitis B has a long incubation period. HEPLISAV‑B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common patient-reported adverse reactions reported within 7 days of vaccination were injection site pain (23%‑39%), fatigue (11%‑17%), and headache (8%‑17%).
There are no adequate and well-controlled studies of HEPLISAV‑B in pregnant individuals. Available data, primarily in individuals who received one dose of HEPLISAV‑B in the 28 days prior to or during pregnancy, do not suggest an increased risk of major birth defects and miscarriage.
It is not known whether HEPLISAV‑B is excreted in human milk. Data are not available to assess the effects of HEPLISAV‑B on the breastfed infant or on milk production/excretion.
Vaccination with HEPLISAV‑B may not result in protection of all vaccine recipients.
ADDITIONAL IMPORTANT INFORMATION
HEPLISAV‑B does not treat liver diseases such as cirrhosis or liver cancer.2
Not all liver cancer is caused by the hepatitis B virus.3
Please see full Prescribing Information.
1. Data on file. Dynavax Technologies Corporation. FDA advisory committee briefing document: HEPLISAV‑B™ (Hepatitis B Vaccine [recombinant], adjuvanted). Presented at: Meeting of the Vaccines and Related Biological Products Advisory Committee; July 28, 2017; Silver Spring, MD. 2. HEPLISAV‑B [package insert]. Emeryville, CA: Dynavax Technologies Corporation; 2024. 3. National Cancer Institute. Liver cancer causes, risk factors, and prevention. Last updated May 15, 2024. Accessed July 15, 2024. https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors