Access templates to inform healthcare teams and patients of important policy changes, vaccine recommendations, and standards of care
The following reference guides highlight decision support tools, chart functionalities, and population health strategies specific to Epic and Cerner software systems that can be leveraged to effectively implement hepatitis B vaccination in millions of adults who are now eligible for immunization
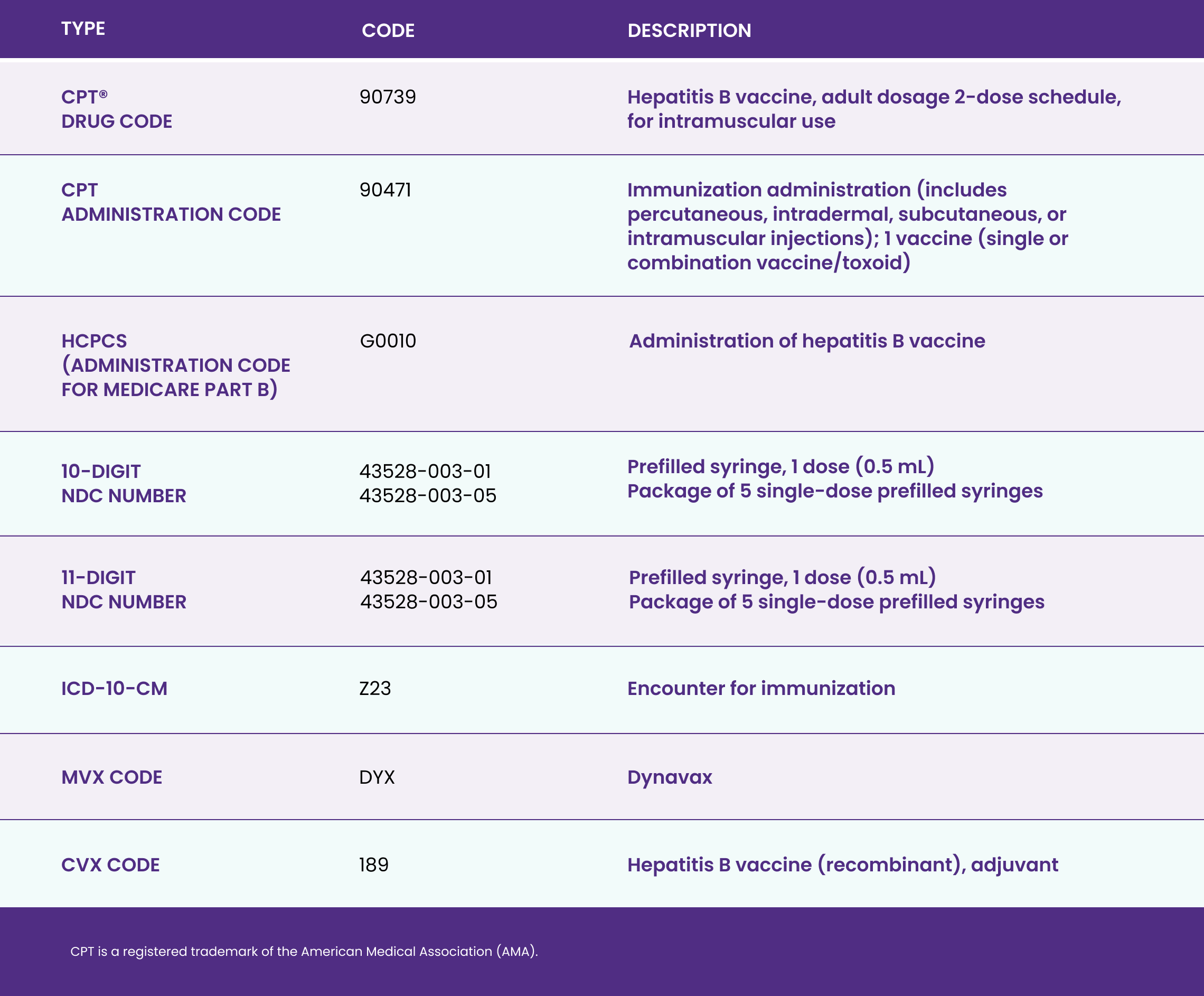
Download to learn more about the only adult hepatitis B vaccine with 2‑dose completion in 1 month1,2
*Coverage and cost may vary and are subject to change without notice. Reimbursement decisions are made by individual insurance plans.
†The Advisory Committee on Immunization Practices recommends vaccines for CDC review and publication; all recommendations are eligible for first-dollar coverage.
Questions about coverage and reimbursement?
Contact HEPLISAV‑B Customer Support
Call 1‑84‑HEPLISAV (1‑844‑375‑4728) Option 3
Monday-Friday 10 am to 4 pm EST
HEPLISAV‑B Customer Support provides:
To report suspected adverse reactions,
Call 1‑84‑HEPLISAV (1‑844‑375‑4728) or contact the FDA at 1‑800‑FDA‑1088 or www.fda.gov/medwatch
You are encouraged to report vaccine adverse events to the US Department of Health and Human Services. Visit www.vaers.hhs.gov to file a report, or call 1‑800‑822‑7967.

ACIP=Advisory Committee on Immunization Practices; CDC=Centers for Disease Control and Prevention; EHR=electronic health record
This site uses cookies to improve your experience. By continuing to use this website you are agreeing to our Cookie Policy.
INDICATION
HEPLISAV‑B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV‑B to individuals with a history of severe allergic reaction (eg, anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV‑B, including yeast.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV‑B.
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to HEPLISAV‑B.
Hepatitis B has a long incubation period. HEPLISAV‑B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common patient-reported adverse reactions reported within 7 days of vaccination were injection site pain (23%‑39%), fatigue (11%‑17%), and headache (8%‑17%).
There are no clinical studies of HEPLISAV‑B in pregnant women. Available human data on HEPLISAV‑B administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
It is not known whether HEPLISAV‑B is excreted in human milk. Data are not available to assess the effects of HEPLISAV‑B on the breastfed infant or on milk production/excretion.
Vaccination with HEPLISAV‑B may not result in protection of all vaccine recipients.
ADDITIONAL IMPORTANT INFORMATION
HEPLISAV‑B does not treat liver diseases such as cirrhosis or liver cancer.1
Not all liver cancer is caused by the hepatitis B virus.4
Please see full Prescribing Information.
1. HEPLISAV‑B [package insert]. Emeryville, CA: Dynavax Technologies Corporation; 2023. 2. Centers for Disease Control and Prevention. Recommended adult immunization schedule for ages 19 years or older. Updated February 29, 2024. Accessed May 9, 2024. https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf 3. Hughes R IV, Maxim R, Fix A. Vague vaccine recommendations may be leading to lack of provider clarity, confusion over coverage. Health Affairs Blog. May 7, 2019. Accessed July 18, 2024. https://www.healthaffairs.org/do/10.1377/forefront.20190506.172246 4. National Cancer Institute. Liver cancer causes, risk factors, and prevention. Last updated May 15, 2024. Accessed July 15, 2024. https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors